amdl elisa dr 70 kit|amdl elisa fdp : manufacture Serum DR-70 levels were measured using the UniPharma-ELISA DR-70 kit (UniPharma Co. Ltd. Taipei, Taiwan) by following the manufacturer’s protocol by the same biochemist. . Wu D, Zhou X, Yang G et al (1998) Clinical performance of the AMDL DR-70 immunoassay kit for cancer detection. J Immunoassay 19:63–72. Article CAS Google Scholar . This symbol is only to be used when there is an accompanying Sterile symbol (5.2.1 to 5.2.5 or 5.2.10). This symbol is not to be used on reusable medical devices that are .
{plog:ftitle_list}
Autoclaves are extremely powerful and can be a bit intimidating to those who are inexperienced with operating them, which is why we’ve put together this guide to standard autoclave working procedure to help you get .
The AMDL, Inc. DR-70® (FDP) assay is an ELISA based assay utilizing removable strips in a .The AMDL-ELISA DR-70 (FDP) test (AMDL Diagnostics, Inc., Tustin, CA) is the first new in .
Serum DR-70 levels were measured using the UniPharma-ELISA DR-70 kit (UniPharma Co. Ltd. Taipei, Taiwan) by following the manufacturer’s protocol by the same biochemist. . Wu D, Zhou X, Yang G et al (1998) Clinical performance of the AMDL DR-70 immunoassay kit for cancer detection. J Immunoassay 19:63–72. Article CAS Google Scholar . Mr. Douglas MacLellan, Chairman and CEO of AMDL stated, Over the past six months AMDL has been executing a very aggressive commercialization strategy for DR-70, which is progressing at a faster pace than originally expected. With several key distribution and collaboration partnerships under our belt, and more in the pipeline, we feel timing is .
The complete article about AMDL-ELISA DR-70 (FDP) can be accessed via AMDL's corporate website located at www.amdl.com under the RESOURCES link. "When we compared the DR-70 immunoassay with conventional tumor markers, DR-70 turned out to be superior to CEA in the detection of patients with colorectal cancer," according to the . DR-70 was evaluated using the AMDL DR-70 kit, which was developed by Super Religare Laboratories, Kolkata. This enzyme-linked immunosorbent assay utilizes affinity purified rabbit anti-DR-70 polyclonal antibodies. . Advantages of the AMDL-ELISA DR-70 (FDP) assay over carcinoembryonic antigen (CEA) for monitoring colorectal cancer patients. J . The DR-70® (FDP) test was the first cancer test cleared by USFDA for monitoring colorectal cancer (CRC) since Carcinoembryonic Antigen (CEA) in 1982 and had 12% to 100% greater positive concordance rates than CEA in this group. The DR-70® (FDP) test was the first cancer test cleared by USFDA for monitoring colorectal cancer (CRC) since Carcinoembryonic . The control group of this study consisted of patients whose total colonoscopy was reported as normal. Results: Serum levels of DR-70 was found to be significantly higher in patients with colorectal cancer than healthy participants (p = 0.001). Receiver operating curve analyses indicated a cut-off value of 1.69 μg/ml for serum DR-70 levels.
The test results showed that the DR-70 immunoassay kit was capable of detecting cancers with high degree of specificity and sensitivity. At 95% specificity level, the sensitivity of the assay was 87.8%, 92.6%, 65.2% and 66.7%, respectively for lung, stomach, breast and rectum cancers. Furthermore the test kits were shown to be stable and .
were analysed using DR-70 immunoassay. The serum DR-70 concentration was measured following the manufacturer’s protocol for the AMDL-ELISA DR-70 kit (AMDL Inc., Tustin, CA, USA). The study protocol was approved by local Ethics Committee of a state training and research hospital (Dr. Lutfi Kirdar Kartal Training and Research Hospital,
According to the manufacturer's instructions, plasma DR‐70 concentrations were measured using a commercial ELISA kit (DR‐70 ELISA, Uni‐pharma Inc., Taipei, Taiwan). 10 , 16 The retail kit contained a microwell plate of 96‐well strips coated with affinity‐purified rabbit anti‐DR‐70 polyclonal antibodies, a horseradish peroxidase . Wu D et al. Clinical performance of the AMDL DR-70 immunoassay kit for cancer using FOBT, this approach should improve the CRC early stage detection. . Small-Howard A et al. Advantages of the AMDL-ELISA DR-70 (FDP) Assay over 10mm) .1 For CRC, FIT has the sensitivity of 50-87%.1 • Blood sample was obtained by venipuncture for each subject . Assay of AMDL DR-70 by enzyme-linked immunosorbent assay (ELISA) . AMDL DR-70 detection kits were bought from the AMDL Corporation, USA. Serum concentrations of AMDL DR-70 were measured in accordance with the manufacturers’ instructions. In short, samples in a dilute buffer were added in 100-μL amounts to wells of an antibody-coated .Supporting: 1, Mentioning: 28 - A clinical study using DR-70 immunoassay for the detection of 13 different cancers have been conducted with 277 healthy subjects and 136 cancer patients. The test results showed that the DR-70 immunoassay kit was capable of detecting cancers with high degree of specificity and sensitivity. At 95% specificity level, the sensitivity of the assay was .
The DR-70 immunoassay reliably differs between gastric cancer and healthy controls, promising to become a useful cancer detection tool in clinical practice. BACKGROUND/AIMS To assess the utility of the DR-70 immunoassay in the diagnosis of gastric cancer. MATERIALS AND METHODS A total of 29 patients with histologically proven . The AMDL-ELISA DR-70 (FDP) test (AMDL Diagnostics, Tustin, CA, USA), which is an immunoassay that measures the FDPs in patient serum, is an in-vitro diagnostic cancer test that has been cleared by . Using this cut-off value, the DR-70 immunoassay showed a good clinical performance with a sensitivity of 91% and a specificity of 93%. Patients with advanced tumour spread showed significantly higher DR-70 values than those with early-stage tumours (P . The AMDL-ELISA DR-70 (FDP) test (AMDL Diagnostics, Inc., Tustin, CA) is the first new in vitro diagnostic cancer test to be cleared by the US FDA for monitoring colorectal cancer (CRC) since January 14, 1982 when Carcinoembryonic Antigen (CEA) was approved. . Wu D., et al. Clinical performance of the AMDL DR-70 immunoassay kit for cancer .
The AMDL-ELISA DR-70 (FDP) test (AMDL Diagnostics, Tustin, CA, USA), which is an immunoassay that measures the FDPs in patient serum, is an in-vitro diagnostic cancer test that has been cleared by . The serum DR-70 concentration was measured following the manufacturer’s protocol for the AMDL-ELISA DR-70 kit (AMDL Inc., Tustin, CA, USA). 2.1. Statistical Analysis. The Statistical Package for Social Sciences (SPSS) for Windows, v. 16.0, was used for all statistical analyses. The normal distribution of the continuous variables was analyzed .(San Diego, CA) May 15, 2009 – GenWay Biotech, Inc., (www.genwaybio.com) a US-based diagnostic company which specializes in providing protein and antibody solutions, announced its partnership with AMDL, Inc., a US-based pharmaceutical company with major operations in China, regarding the distribution of AMDL's DR-70 (FDP) cancer test in both .
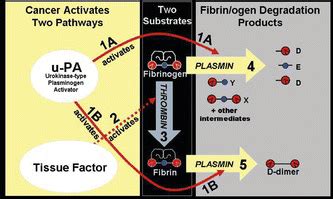
Aim: The purpose of this study was to determine the diagnostic potential of DR-70 assay in patients with colorectal cancer and to investigate the relationship between serum DR-70 levels and the biological characteristic of the tumor. Patients & The serum DR-70 concentration was measured following the manufacturer’s protocol for the AMDL-ELISA DR-70 kit (AMDL Inc., Tustin, CA, USA). 2.1. Statistical Analysis. The Statistical Package for Social Sciences (SPSS) for Windows, v. 16.0, was used for all statistical analyses. The normal distribution of the continuous variables was analyzed . US-based pharmaceutical company Radient Pharmaceuticals Corp announced the Journal of Immunoassay and Immunochemistry (April 2010, Volume 31) has published a peer-reviewed journal article written by Dr Andrea Small-Howard and Mr. Holden Harris titled "Advantages of the AMDL-ELISA DR-70 (FDP) Assay Over Carcinoembryonic Antigen (CEA) .
Human DR-70TM(Tumor Marker DR-70 For Lung Cancer) ELISA Kit . Catalog No.: MBS762031. Size: 48T/96T . Reactivity: Human . Range: 0.156-10ug/ml . Sensitivity: 0.094ug/ml . Application: For quantitative detection of DR-70TM in serum, plasma, tissue homogenates and other biological fluids. Storage: 4°C for 6 months . Principle: Sandwich . NOTE .J Immunoassay 19:63–72CrossRef Wu D, Zhou X, Yang G et al (1998) Clinical performance of the AMDL DR-70 immunoassay kit for cancer detection. J Immunoassay 19:63–72 CrossRef. 22. go back to reference Li X, Qiao Z, Long X, Wei J, Cheng Y (2005) Serum concentration of AMDL DR-70 for the diagnosis and prognosis of carcinoma of the tongue. .
dr 70 fdp
amdl elisa fdp
The AMDL-ELISA DR-70 (FDP) test (AMDL Diagnostics, Inc., Tustin, CA) is the first new in vitro diagnostic cancer test to be cleared by the US FDA for monitoring colorectal cancer (CRC) since .
Name of the test was Onko-sure (DR-70 Elisa Test) and manufactured at Tustin, California, USA by AMDL Diagnostics & Jaiva Technologies, Inc. Test kit batch number was DR 2101193. This is an ELISA based assay, which uses affinity purified rabbit antiDR-70 polyclonal antibodies coated 96 micro titer plate in a well format.The AMDL-ELISA DR-70 (FDP) test (AMDL Diagnostics, Tustin, CA, USA), which is an immunoassay that measures the FDPs in patient serum, is an in-vitro diagnostic cancer test that has been cleared by .
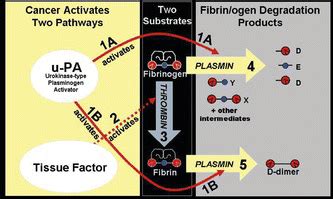
Hu-Friedy offers a quick and accurate approach to steam sterilizer biological monitoring. SporeCheck™ is simple to use: one biological indicator vial works for all types of steam sterilizers, including gravity, prevacuum, and flash.
amdl elisa dr 70 kit|amdl elisa fdp